top of page
.png)

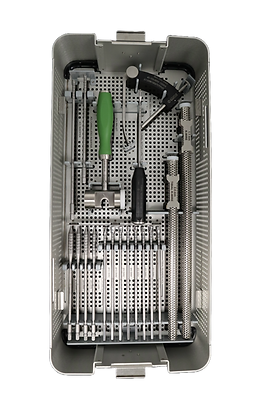
Instrumentation
.png)
.png)
A
B
C
D
E
F
H
I
J
K
L
M
G
N
Resources
A. Femoral K-wire
· 2.4 mm
B. Tendon Sizer
· 6, 7, 8, 9, 10, 11, 12 mm
C. Quick Connect Handle
· 1/4"
D. Sizing Tubes
· 8, 9, 10 mm
H. Tibial Drill Guide
I. ACL Mallet
J. ACL Inserter
· Regular and cannulated
K. Tunnel Notch Curette
L. K-Wires
· 1.1, 2.4 (tibial) mm
Available in the following sizes: 7x23, 7x28, 8x23, 8x28, 9x23, 9x28, 9x33, and 10x23 mm
Special Order Only: 11x28, 11x33, 12x28, and 12x33 mm
CITRELOCK ACL
®
Soft Tissue Fixation Device
CITRELOCK ACL is designed as a soft fluted, self-locking device. It features a cannula that passes through this central axis to help align with the surgically prepared site. The multi-lead, high-pitch design reduces screw revolutions and mitigates tendon laceration or twisting during insertion.
E. BTB Pliers
· 9, 10 mm
F. Inserters (2)
G. Femoral Reamers
· 7, 7.5, 8, 9, 9.5 10, 10.5 mm
M. Tibial Reamers ACL
· 7.5, 8, 8.5, 9, 9.5, 10, 10.5 mm
N. Dilators ACL
· 7, 8, 9, 10 mm
Instructions for Use
Videos
bottom of page




